
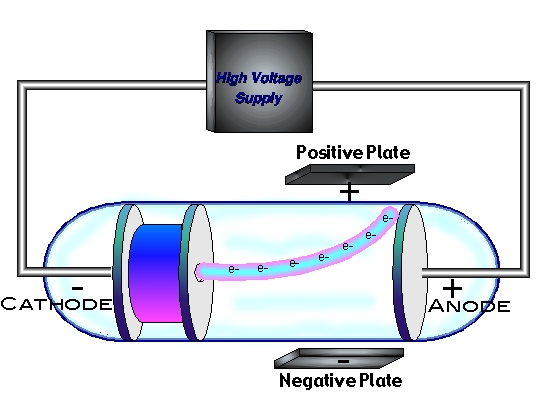
In fact, for almost 100 years, it seemed as if Dalton's Atomic Theory was the whole truth. Instead, the theory should suggest new experiments that can be performed to test whether or not the original theory is accurate and complete.ĭalton's Atomic Theory held up well to a lot of the different chemical experiments that scientists performed to test it. The scientific method, though, doesn't stop once a theory has been proposed. With these observations (which came from careful measurement), Dalton proposed his Atomic Theory – a model which suggested how the underlying structure of matter might lead to the three observations above. When elements form more than one compound, the different masses of one element that are combined with the same mass of the other element are in a ratio of small whole numbers.Elements always combine in the same proportions by mass when they form a given compound.Mass is neither created nor destroyed during a chemical reaction.Do you remember the scientific method introduced in early on in the book? Chemists using the scientific method make careful observations and measurements and then use these measurements to propose theories. But Dalton's Atomic Theory isn't the end of the story. In the last lesson, you learned about the atom, and the early experiments that led to the development of Dalton's Atomic Theory.

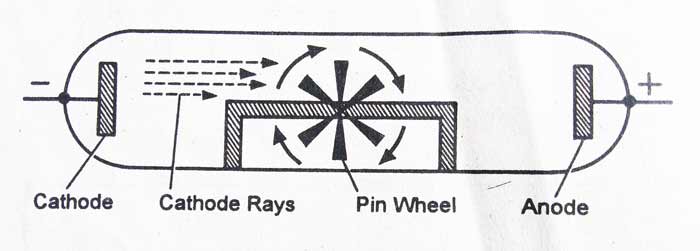
6 Rutherford Suggested Electrons "Orbited".3 Protons Were Thought to Exist but Discovered Much Later.2 Thomson Discovered Electrons Were Part of the Atom.


 0 kommentar(er)
0 kommentar(er)
